A representative sampling of tuna muscle for mercury control
Accepted: 25 September 2020
HTML: 42
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
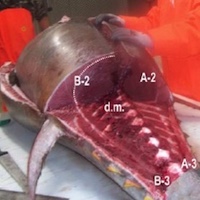
The mechanisms of mercury accumulation and distribution in fish tissues are related to its high affinity for sulfhydryl groups in proteins. There is evidence that mercury is distributed unevenly based on the different reactivity of these groups in the various muscle proteins. Tuna fish also shows numerous specialized anatomical features including the structure of the swimming muscles and some form of endothermy, which generates variations in the mercury content between dark and white muscle and between muscle tissues with different lipid content. The aim of the study is to verify, through a suitable sub lot of Thunnus thynnus caught by a static trap in south-western Sardinia, the effective uneven distribution of mercury in the various muscles and also identify the sites representative of the entire carcass. In agreement with other authors, the results show that even in the Bluefin tuna of the Mediterranean, the site “anterior extremity of upper loin (schienale in Italian)” is representative of the mercury average content of muscle tissues as a whole.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ijfs.2020.9055
https://doi.org/10.4081/ijfs.2020.9055





