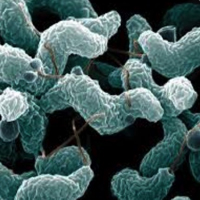
Detection and quantification of Campylobacter in foods: New analytic approaches to detect and quantify Campylobacter spp. in food samples
Submitted: 30 September 2019
Accepted: 7 January 2020
Published: 28 August 2020
Accepted: 7 January 2020
Abstract Views: 1151
PDF: 640
HTML: 58
HTML: 58
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Similar Articles
- Maria Francesca Peruzy, Immacolata La Tela, Maria Rosaria Carullo, Simona Ioele, Yolande Thérèse Rose Proroga, Anna Balestrieri, Nicoletta Murru, Occurrence and distribution of Salmonella serovars associated with human infection isolated from irrigation waters and food-producing animals in southern Italy: eleven-year monitoring (2011-2021) , Italian Journal of Food Safety: Vol. 12 No. 4 (2023)
You may also start an advanced similarity search for this article.

 https://doi.org/10.4081/ijfs.2020.8591
https://doi.org/10.4081/ijfs.2020.8591



