Effects of inhibition of lymphangiogenesis by the vascular endothelial growth factor receptor 3 (VEGFR-3) inhibitor, MAZ51 on full thickness wounds in mice
Accepted: 22 February 2021
HTML: 77
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
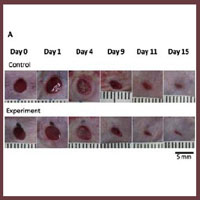
We herein used MAZ51 to inhibit lymphangiogenesis and aimed to clarify the effect of inhibition of lymphangiogenesis on wound healing. BALB/c male mice were divided into two groups: the control group which was injected the dimethyl sulfoxide (DMSO), the experiment group was injected MAZ51 in the DMSO. All wounds were observed for 15 days and the wound areas were measured. Tissue samples were harvested on day 3, 7, 9, 11, 13 and 15, and subjected to immunostaining of blood vessels and lymphatic vessels. There are no significant differences between two groups in the wound area, the number of blood vessels and lymphatic vessels. The number of blood vessels peaked on day 7 in both groups as with previous studies, while the number of lymphatic vessels peaked on 13 or 15 in both groups. This result revealed delayed lymphangiogenesis in comparison with previous studies. The wound healing process in the control and experiment groups were similar, but both groups seemed delayed lymphangiogenesis comparing with previous studies. Injections of MAZ51 or/and DMSO did not affect angiogenesis, while they may affect lymphangiogenesis.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/vl.2021.9385
https://doi.org/10.4081/vl.2021.9385





