 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Effect of selected disinfectants on biofilm-forming clinical isolates of Staphylococcus aureus in Lagos State, Nigeria
Accepted: 11 September 2023
HTML: 26
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
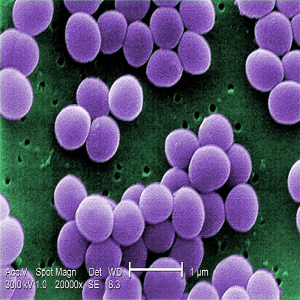
Background and Aims: Staphylococcus aureus is one of the most important pathogens of public health concern and a leading cause of nosocomial infections. In this study, we evaluated the effect of routinely used disinfectants in hospitals for surface decontamination on biofilm-forming S. aureus.
Materials and Methods: forty-eight S. aureus isolates were phenotypically evaluated for biofilm formation using the Tissue Culture Plate (TCP) technique. Effect of disinfectants (Dettol®, Izal®, Jik® and Savlon®) on biofilm was tested and time-kill kinetics evaluated. PCR was used to confirm the identity of S. aureus using species-specific primers.
Results: biofilm formation assay revealed that 15 (31.2%) of the isolates formed biofilm with 7 (14.5%) and 8 (16.6%) considered as strong and moderate biofilm formers, respectively. Biofilm formation was time-dependent (p<0.0001). Jik® was significantly effective (p<0.0001) as it disrupted biofilm formed in all 15 (100%) isolates, followed by Izal® 13 (86.6%), Savlon® 11 (73.3%) and Dettol® 9 (60%). Time-kill kinetics of the four disinfectants revealed Dettol®, Jik® and Savlon® achieved total (100%), (7 log10) lethality against isolates within 1 h contact time while Izal® attained complete lethality at 6 h contact time.
Conclusions: of the four disinfectants evaluated Jik®, a chlorine- based formulation, was more effective in destroying biofilmforming S. aureus. The need to use effective disinfectants in sanitization is imperative to facilitate the control and prevention of hospital and community-acquired infections.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/mm.2023.11445
https://doi.org/10.4081/mm.2023.11445



