Ovine fetuses from slaughterhouses: A useful source for neural cell primary cultures
Accepted: April 7, 2021
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
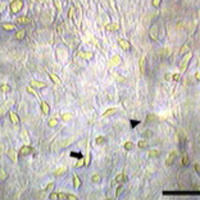
A lot of evidence demonstrates that sheep could represent an experimental model to set up medical procedures in view of their application on humans. Sheep are chosen as models for human biomechanical studies because their skeleton has some similarities to humans. The aim of this work was to set up sheep primary cultures from ovine fetuses at different ages, from pregnant uteri retrieved at local abattoirs. Cell characterization showed that one cell population was immunopositive to GFAP and identifiable as astrocytes, whereas a second cell type was III β-tubulin-positive, and hence classified as neurons. At 60-days old fetus is suitable to obtain neurons, whereas in a 90-days old fetus the cell culture is predominantly characterized by glial cells. The procedure here proposed is inexpensive, in fact, collecting fetuses during sheep slaughtering is a cost-saving option, unlike common experimental animals such as mice, rats, rabbits, that require very high economical efforts. Finally, our protocol fully eliminates the need of animal killing, being living animals replaced by a validated in vitro model in agreement with the 3Rs statement.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/jbr.2021.9344
https://doi.org/10.4081/jbr.2021.9344



