Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Evaluation of Short Chain Fatty Acids (SCFAs) intestinal absorption, following digestion and fermentation of a novel medical device containing partially-hydrolyzed Guar gum plus simethicone
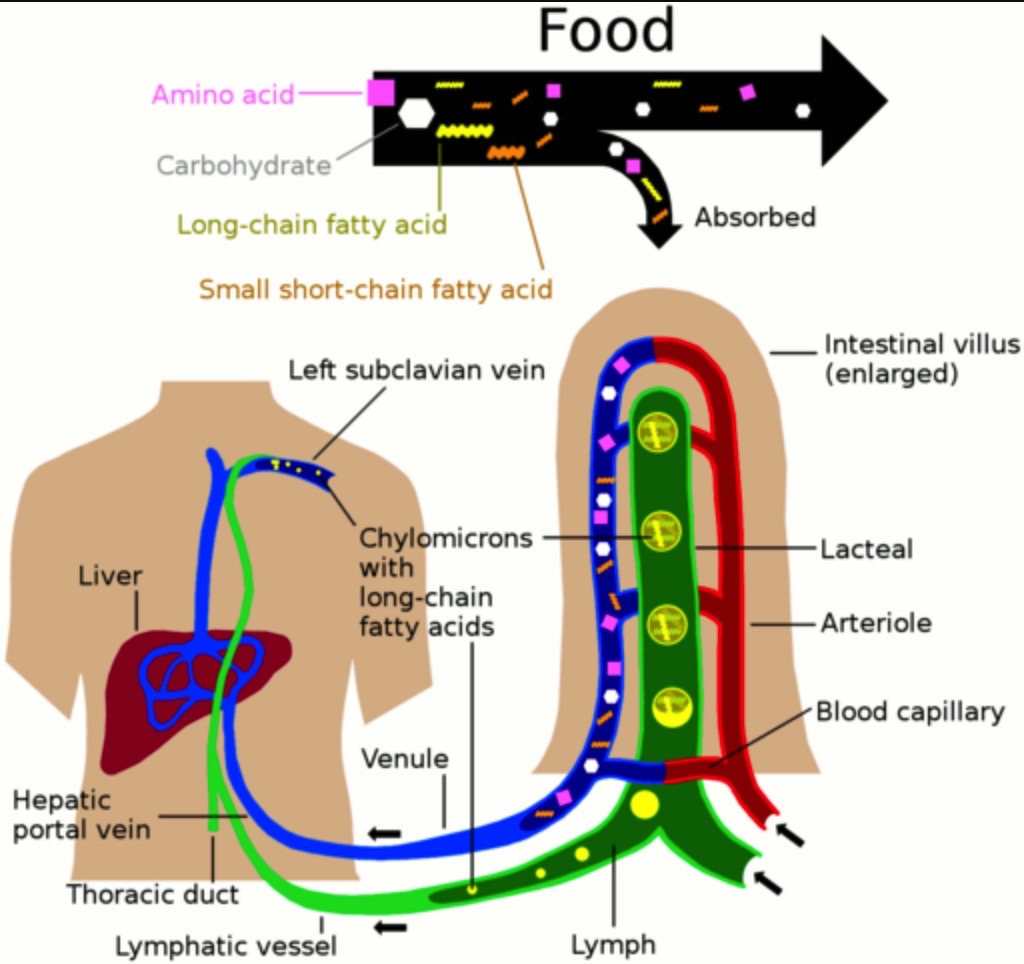
Irritable Bowel Syndrome (IBS) is a common disease characterized by alternate symptoms (diarrhea and constipation) and intestinal gas overproduction. A new medical device (Fibergone®), containing Partially Hydrolyzed Guar Gum (PHGG) and Simethicone (SM) has been proposed for managing patients with bowel disorders. PHGG acts also as a prebiotic so increasing the Short-Chain Fatty Acid (SCFA) production, useful for intestinal physiology. This in vitro study investigated the effects exerted by PHGG+SM on SCFA production and their intestinal absorption following in vitro digestive process and fermentation model. An in vitro model evaluated the digestive process and fermentation using simulated digestive fluids and a human intestinal epithelium in vitro model derived from based on intestinal adenocarcinoma Caco-2 cells (ATCC, HTB-37TM) and organized as a functional monolayer on Transwell® inserts. PHGG+SM was added in experiments and compared with a control (non-treated). SCFA production and absorption were assessed. Viability and barrier integrity of the intestinal epithelium model were also evaluated. PHGG+SM significantly (p<0.05) increased SCFAs content after fermentation, indicating that this medical device is effectively fermented at the large intestine level. However, in relation to SCFAs bioavailability, their absorption did not increase compared to the non-treated condition in the light of the physiological contribution of SCFAs resulting from the microflora. PHGG+SM did not affect intestinal epithelium apparent permeability (Papp) and viability. This in vitro study documented that partially hydrolyzed guar gum combined with simethicone significantly affects short-chain fatty acids production and consequently could be fruitfully employed in managing patients with intestinal disorders.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/jbr.2023.11154
https://doi.org/10.4081/jbr.2023.11154