Kearns-Sayre syndrome with optic nerve atrophy phenotype: A possible biological and clinical concurrence of two mutations?
Accepted: January 30, 2022
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
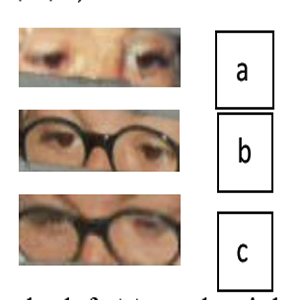
The authors report about the association of progressive external ophthalmoplegia, atypical pigmentary retinopathy, ataxia phenotype with onset in first months of life (Kearns-Sayre syndrome) and with optic nerve atrophy and deafness. The localization of retinal lesions was coincident with that reported by multifunctional electroretinogram (mfERG) in OPA 1 mutation. The authors hypothesize that Kearns-Sayre mitochondrial mutation may be associated with OPA 1 missense mutation, with worsening of symptomatology, as occurs in the reported case. The prolonged rehabilitation and treatment with coenzyme Q10 for many years gave positive results, with amelioration of ophthalmoplegia, stopping of aggravation of retinal damage and optic nerve atrophy, maintaining of vision some meters away, possibility of socialization and proprioceptive ability amelioration.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/jbr.2022.10308
https://doi.org/10.4081/jbr.2022.10308



