Gut microbiota shift of spangled emperor under pollution stress
Accepted: April 27, 2021
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
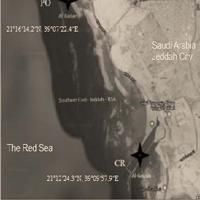
Hepatic antioxidant enzymes as oxidative stress biomarkers were investigated and correlated with the identified dominant gut microbial phyla. The results showed that while the antioxidant enzymes, Superoxide Dismutase (SOD), and Catalase (CAT) levels were reduced in the polluted PO site, significant elevation (*P ≥ 0.05) was observed at the clean reference CR site indicating negative correlation to pollution stress. On the other hand, among five significant bacterial genera, Lactobacillus and Vagococcus showed a positive relationship to the oxidative pollution stress between PO and CR sites. Diversity and bacterial richness had been observed in the PO site compared to the CR site. As a result, 429,346 sequences were obtained from the pooling of 20 samples identified into 10 phyla and 79 genera in which Firmicutes was dominant in both PO and CR sites. The number of common OTUs was 221 for both CR and PO samples. The results revealed that under the stressed environmental state, the homo-lactic Vagococcus genus is dominant over the hetero-lactic Lactobacillus, which uses less energy in the derived process.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/jbr.2021.9519
https://doi.org/10.4081/jbr.2021.9519



