Evaluate the Therapeutic Effect of Allicin (L-cysteine) on Clinical Presentation and Prognosis in Patients with COVID-19
Accepted: 18 April 2021
HTML: 35
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
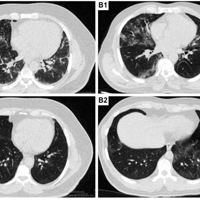
The antiviral effectiveness of allicin (L-cysteine) has been shown by numerous studies in both levels of clinical and animals. The aim of this study was to evaluate the therapeutic effect of allicin (L-cysteine) on clinical presentation and prognosis. In the current study, 66 patients with COVID-19 based on clinical, radiological presentations and RT-PCR results, were enrolled in two groups of placebo and allicin. In the both allicin (L-cysteine) and placebo groups (n=33 in each group), the capsules were prescribed two times a day for two weeks. Clinical signs and symptoms, blood parameters and chest CT scan were evaluated before and two weeks after treatment. The results showed that allicin (L-cysteine) could significantly impact on improvement of signs and symptoms of COVID-19 after two weeks of treatment in comparison to placebo. Allicin (L-cysteine) not only improve the clinical signs, but also ameliorate the lab and radiological data, which suggest a therapeutic effect for this agent in COVID-19. Our data suggest the therapeutic effect of allicin (L-cysteine) on COVID-19 through improvement of clinical symptoms and acceleration of the healing process.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2021.9518
https://doi.org/10.4081/ejtm.2021.9518



