Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Correlation between ETFDH mutations and dysregulation of serum myomiRs in MADD patients
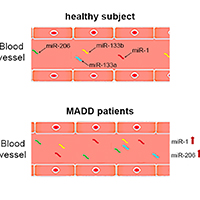
Multiple acyl-CoA dehydrogenase deficiency (MADD) is a rare fatty acids oxidation disorder which is often associated with deficiency of electron transfer flavoprotein dehydrogenase (ETFDH). In this study we reported clinical features and evaluation of expression profile of circulating muscle-specific miRNAs (myomiRs) in two MADD patients carrying different ETFDH gene mutations. Patient 1 was a compound heterozygote for two missense mutations. She showed a late onset MADD clinical phenotype and a significant increase of serum myomiRs. Patient 2, carrying a missense and a frameshift mutation, displayed early onset symptoms and a slight increase of some serum myomiRs.
Downloads
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2019.8880
https://doi.org/10.4081/ejtm.2019.8880