Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Muscle hypertrophy and muscle strength: dependent or independent variables? A provocative review
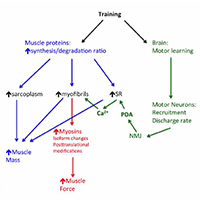
The question whether the muscle hypertrophy induced by resistance training, hormone administration or genetic manipulation is accompanied by a proportional increase in muscle strength is still open. This review summarizes and analyses data obtained in human and rodent muscles in studies that have monitored in parallel changes in muscle size and changes in muscle force, measured in isometric contractions in vivo, in isolated muscles ex vivo (in rodents) and in single muscle fibers. Although a general positive relation exists among the two variables, a number of studies show a clear dissociation with increase of muscle size with no change or even decrease in strength and, vice versa, increase in strength without increase in size. The possible mechanisms of such dissociation, which involves neural motor control and/or cellular and molecular adaptations of muscle fibers, are briefly discussed.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2020.9311
https://doi.org/10.4081/ejtm.2020.9311