Wound healing effects of Persian walnut (Juglans regia L.) green husk on the incision wound model in rats
Accepted: 22 December 2019
HTML: 27
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
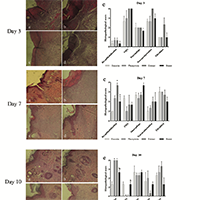
Walnut green husk (WGH) has been mentioned as a wound-healing agent in traditional Iranian medicine. Although previous studies indicated that WGH is a good source of pharmaceutical ingredients, they did not assess its wound healing activity; so the present study set out the scientific validation of the wound healing potential of the Persian walnut. Total phenolic content, reducing power, DPPH, and nitric oxide scavenging activity of aqueous ethanol extract of WGH was evaluated. Forty-eight male Wistar albino rats were divided into four groups of 12 each. An incision wound was created on the dorsal region of each rat. WGH extract (20% w/w), WGH burnt residues (20% w/w), Eucerin, and Phenytoin ointments were used in each group. Wound length, contraction percentage, and histopathological evaluations were recorded on days 3, 7, 10, and 14. Total phenolic content and EC50 values of reducing power, DPPH and nitric oxide scavenging activity of the WGH extract were 61.34 ± 0.64 mg/g dry extract, 0.95 ± 0.02 mg/mL, 0.35 ± 0.01 mg/mL, and 0.28 ± 0.01 mg/mL, respectively. Treated animals with WGH extract showed significantly (p ≤ 0.05) better results for physical and pathological parameters compared to the control group; overall, WGH extract showed better results than WGH burnt residues. The present study indicated that the WGH aqueous ethanol extract has a promising potential for wound healing in the animal model and could be a valuable resource for developing new wound-healing medicines for humans.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2019.8671
https://doi.org/10.4081/ejtm.2019.8671



