Angiotensin-(1-7) improves skeletal muscle regeneration
Accepted: 21 November 2023
HTML: 28
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
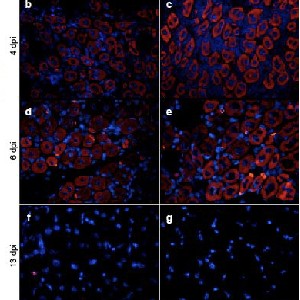
Skeletal muscle possesses regenerative potential via satellite cells, compromised in muscular dystrophies leading to fibrosis and fat infiltration. Angiotensin II (Ang-II) is commonly associated with pathological states. In contrast, Angiotensin (1-7) [Ang-(1-7)] counters Ang-II, acting via the Mas receptor. While Ang-II affects skeletal muscle regeneration, the influence of Ang-(1-7) remains to be elucidated. Therefore, this study aims to investigate the role of Ang-(1-7) in skeletal muscle regeneration. C2C12 cells were differentiated in the absence or presence of 10 nM of Ang-(1-7). The diameter of myotubes and protein levels of myogenin and myosin heavy chain (MHC) were determined. C57BL/6 WT male mice 16-18 weeks old) were randomly assigned to injury-vehicle, injury-Ang-(1-7), and control groups. Ang-(1-7) was administered via osmotic pumps, and muscle injury was induced by injecting barium chloride to assess muscle regeneration through histological analyses. Moreover, embryonic myosin (eMHC) and myogenin protein levels were evaluated. C2C12 myotubes incubated with Ang-(1-7) showed larger diameters than the untreated group and increased myogenin and MHC protein levels during differentiation. Ang-(1-7) administration enhances regeneration by promoting a larger diameter of new muscle fibers. Furthermore, higher numbers of eMHC (+) fibers were observed in the injured-Ang-(1-7), which also had a larger diameter. Moreover, eMHC and myogenin protein levels were elevated, supporting enhanced regeneration due to Ang-(1-7) administration. Ang-(1-7) effectively promotes differentiation in vitroand improves muscle regeneration in the context of injuries, with potential implications for treating muscle-related disorders.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2023.12037
https://doi.org/10.4081/ejtm.2023.12037




