Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Immunoexpression of p53 mutant-type in Iranian patients with primary and recurrence oral squamous cell carcinoma
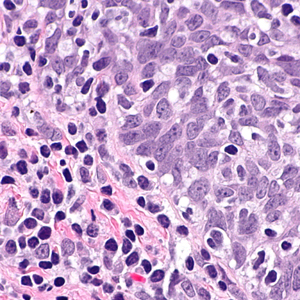
Mutations in tumor suppressor p53 protein can occur at different phases of malignant transformation and affect the patient's prognosis. This study aimed to evaluate the expression of mutant p53 protein in Iranian patients with the primary and recurrence oral squamous cell carcinoma (OSCC). This retrospective cross-sectional study conducted on a group of patients with the primary OSCC (n=122) and the control subjects with oral noncancerous reactive lesions (n=80). Immunohistochemistry was performed with the DO-7 monoclonal antibody against p53 protein, and samples with ≥10% immunostaining were considered positive. Statistical analyses were carried out using SPSS. Positive staining for p53 was observed in none of the control subjects and 57.4% (70 of 122) of the primary OSCC patients (p<0.0001, OR=107.69, 95%CI=6.49-179.0). The p53 immunopositivity had no significant differences between males and females (54.2% vs. 62%, p=0.390), but significantly different between those aged below and over 50 years (p<0.0001, OR=4.52, 95%CI=1.07-12.05). During follow-up, OSCC recurrence occurred in 104 patients, but the phenotype of the mutant p53 protein in patients who relapsed was the same as in matched primary tumors (p=0.763). Risk of recurrence had no significant differences between p53-positive and p53-negative cases (p=0.953), males and females (p=0.263), and age below and over 50 years (p=0.223). Despite its confirmed diagnostic value, the immunoexpression of the p53 mutant protein in OSCC in cancer recurrence was the same as in the primary tumor. However, further studies with a larger sample size and longer follow-up are needed to confirm or change our conclusions.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2022.10847
https://doi.org/10.4081/ejtm.2022.10847