Better isolation, proliferation and differentiation of human adipose-derived mesenchymal stem cells using human serum
Accepted: 30 November 2022
HTML: 30
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
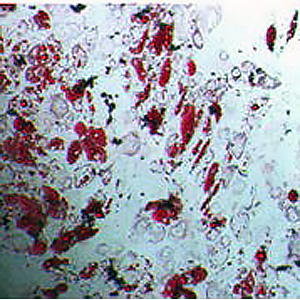
Mesenchymal stem cells have many applications in medicine. Attention to the proliferation and differentiation of stem cell differentiation is an important issue. The aim of this study was to investigate the possibility of optimal isolation, proliferation, and differentiation of adipose tissue-derived mesenchymal stem cells (ADSCs) using human serum. Human serum (HS) was obtained from the venous blood of eight healthy individuals. The rate of proliferation and differentiation of ADSCs and expression of surface markers was assessed by flow cytometry. Bone differentiation was assessed using Alizarin Red staining. Data were analyzed using statistical software. Over time, HS showed more proliferation than fetal bovine serum (FBS) -enriched cells (p <0.05). Differentiation of ADSCs cells ls in HS-enriched medium is faster and more pronounced than differentiation in the control group. The expression of surface markers in the medium containing HS was the same as the medium containing FBS where the expression levels of CD105 and CD95 were found to be positive and the expression of CD34 and CD45 was negative. Due to the better proliferation of adipose tissue-derived mesenchymal cells in the medium containing HS than FBS, it is suggested that human serum be used in future clinical studies. Also, HS is healthier, safer, more accessible, and more affordable than FBS.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2023.10834
https://doi.org/10.4081/ejtm.2023.10834



