Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
Immediate to short-term inflammatory response to biomaterial implanted in calvarium of mice
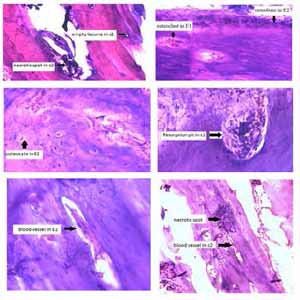
Scaffolds made of biodegradable materials play a very important role in repairing bone defects. Our study was conducted with the aim of investigating inflammation, vascular changes, and tissue necrosis after the placement of 3D printed scaffolds composed of beta-tricalcium phosphate (TCP-β) on the calvarial bone defect of mice. Eight samples of scalp tissue in mice were examined in four groups (one-week control, two-week control, one-week experiment, and two-week experiment). Mice with routine bone defects were selected as the control group and mice with bone defects with β-TCP scaffolds were selected as the experimental group (TCP). The groups were evaluated in terms of inflammatory cells, osteoblast and osteoclast cells, vascular changes, and the number of resorption pit and empty lacuna. The results demonstrated a decrease in inflammatory cells and an increase in osteoclast and osteoblast cells in bone defect sites placed with TCP-β scaffolds (p<0.05). The results of histological staining showed pit resorption and further vascularization in the place of TCP-β scaffolds, but these changes were not statistically significant (p>0.05). Examining the number of empty lacunae in the bone defect site showed that TCP-β could significantly reduce the number of these lacunae in the bone defect sites placed with TCP-β scaffolds (p<0.05). 3D printed scaffolds composed of TCP-β that were implanted in bone defect sites were effective in reducing the inflammatory responses, emptying lacunae and increasing bone regeneration.
Downloads
Citations
10.3390/app14093821
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2022.10785
https://doi.org/10.4081/ejtm.2022.10785