The potential effect of leukocyte filtration methods on erythrocyte-derived microvesicles: One step forward
Accepted: 18 July 2022
HTML: 10
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
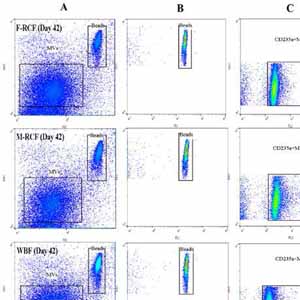
By harmonizing the pre-preparation conditions and also removing some donors’ variations, the current study took one step forward to investigate whether different leukocyte filtration sets influence the quality of RBCs throughout the storage time. Twelve whole blood units were collected, and each unit was split into three equal parts. Thirty-six divided bags were filtered using three different leukocyte-filtration sets including Red Cell and Whole Blood Filters (12 units per filter). The prepared RBCs were refrigerated for up to 42 days and assessed for microvesicle count and size, clotting- and prothrombin time, hemolysis index, and biochemical parameters. A significant increment in erythrocytes microvesicle count (EMVs/μL) was observed during the time in the three filtration sets. The number of EMVs in WBF-RBCs was higher (~1.6 fold) than in F-RCF on day 42 (p=0.035). Interestingly the median fluorescence intensity of EMVs decreased during the storage. The size of MVs rose during the time without any significant differences among the filters. Coagulation time decreased in RBCs over the storage, with no significant differences among the filters. Hemolysis index and lactate concentration increased while glucose level decreased significantly throughout the time. The changes in WBF-RBCs were more drastic rather than RCF-RBCs. The only significant difference in the count of EMVs was between WBF and F-RCF components on day 42. Though the changes in WBF products were more drastic, all the values fell within the standard limits. Accordingly, all three filtration sets can be considered.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2022.10708
https://doi.org/10.4081/ejtm.2022.10708



