Immodulin peptides influence musculoskeletal homeostasis by linking extracellular cues to macrophage and myoblast nuclear receptors
Accepted: 8 September 2022
HTML: 10
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
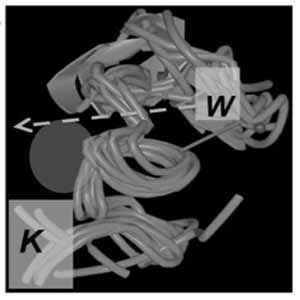
Immodulins are synthetic peptides derived from the C-terminal domains of insulin-like growth factor binding proteins (IGFBPs). Immodulins from the 3/5/6 (but not 1/2/4) IGFBP evolutionary clade transduce extracellular matrix (ECM) signals to RXR, NR4A1 and PPAR-alpha nuclear receptors (NRs) to stimulate novel macrophage lineages. The rationale of this study was to reconcile physical associations of immodulins with ECM and NRs, effects of siRNAs and chemical inhibitors in vivo, and immodulin-driven pro-differentiation effects in cell culture. When added to THP1D cells, immodulins stimulate CD169+ Clec9a+ and Clec12a+ macrophage lineages via a EP300/RXRγ/Nur77 transcriptional mechanism. This phenomenon is accompanied by the secretion of CCL22, IL-10 and TGFbeta and the ability to stimulate FoxP3+ T-cells in co-culture. ECM ligands of 3/5/6 immodulins include iron, zinc, glycosaminoglycans, transferrin and phosphatidylinositol-4,5,-biphosphate (PIP2), which can influence their pro-differentiation effects. Remarkably, immodulins also stimulate myogenesis in C2C12 myoblasts, thereby revealing a novel link between immune and musculoskeletal homeostasis. Distinct NR agonists stimulate these companion differentiation processes. Using solution NMR to guide design, immodulins with a tripeptide extension near the iron-binding pocket demonstrated higher iron-binding and improved pro-differentiation activities. Transferrin-bound immodulin shows binding preference for both high-molecular-weight hyaluronan (HMWHA) and HMWHA:CD44 complexes at endosomal pH, and interacts with PIP2 at normal physiological pH, offering intriguing mechanistic insights.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2022.10695
https://doi.org/10.4081/ejtm.2022.10695



