Uremic encephalopathy: A definite diagnosis by magnetic resonance imaging?
Accepted: 18 June 2022
HTML: 44
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Authors
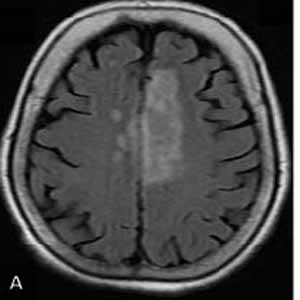
The aim of this study was to investigate the magnetic resonance imaging (MRI) findings for the diagnose uremic encephalopathy and describe the usefulness of MRI findings in the ultimate diagnosis of uremic encephalopathy (UE). A total of 20 patients with uremic encephalopathy admitted to the hospital were evaluated in this prospective study. The clinical manifestations, laboratory and MRI imaging findings, demographic information, and clinical outcome were analyzed for each patient. We observed that the 20 prospectively reviewed patients with UE had no involvement of the basal ganglia or the lentiform fork sign (LFS). However, two-thirds of the patients had white matter involvement, and 80% of the subjects had cerebral or cortical atrophy. The arterial blood gas (ABG) analysis revealed that 50% of the patients suffered from metabolic acidosis (n=10). The results of the present study demonstrated that although the observation of Lentiform Fork Sign and Basal Ganglia involvement in MRI of UE patients is a specific finding the absence of which does not rule out UE. Thus, simultaneous examination of clinical manifestation and laboratory test analyses, along with imaging findings, should also be taken into account.
How to Cite
PAGEPress has chosen to apply the Creative Commons Attribution NonCommercial 4.0 International License (CC BY-NC 4.0) to all manuscripts to be published.

 https://doi.org/10.4081/ejtm.2022.10613
https://doi.org/10.4081/ejtm.2022.10613



